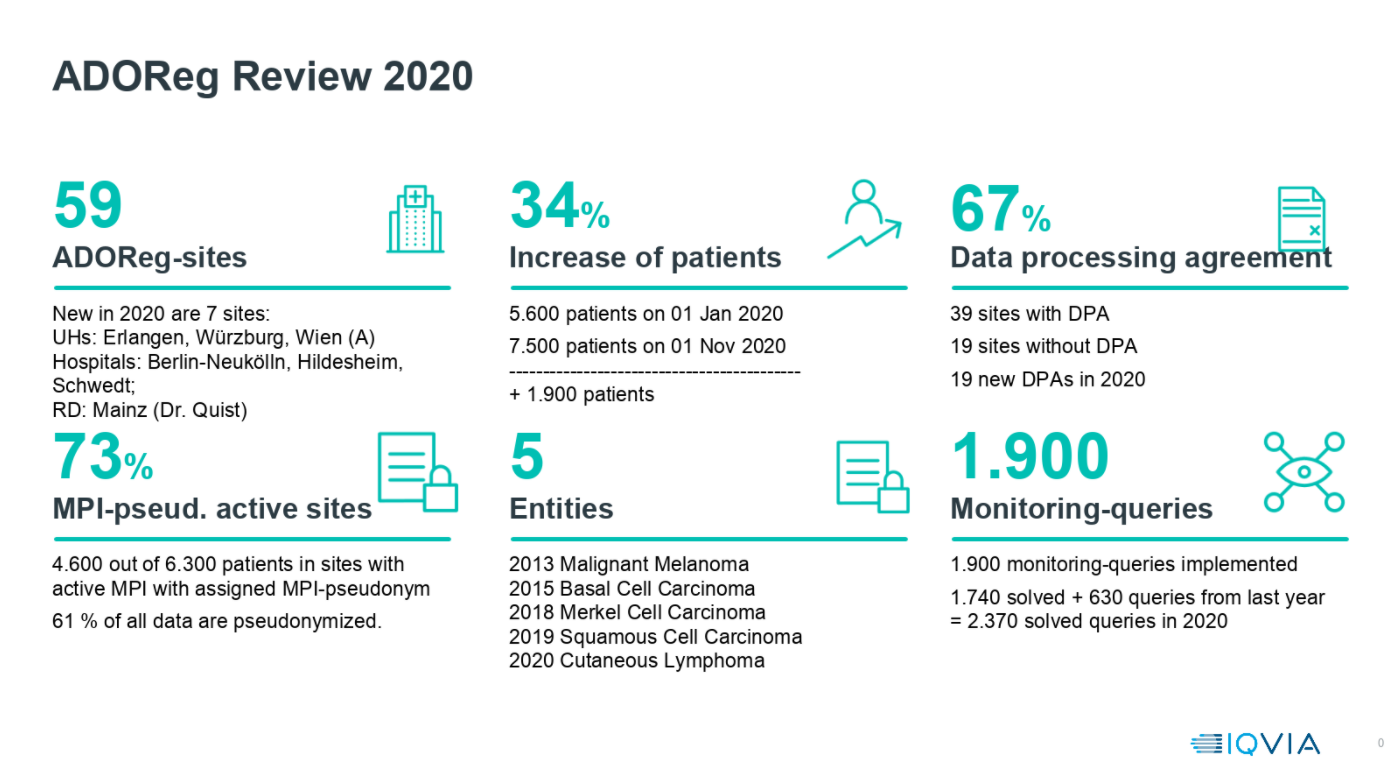
-
ADOReg is the largest prospective clinical registry in Germany in the field of dermatological oncology with great added value for all stakeholders in the healthcare system
More than 75% of the nationally certified skin tumour centres in Germany already participate in the ADOReg and form a steadily growing and established network of recognised experts with deep professional competence. The ADOReg enables the collection and implementation of high-quality real world evidence studies for care and contract research.
Review 2020
Please click on the illustrations for an enlarged view.
Site-based Observational Studies
Implementation and execution of mono- or multi-centre non-interventional studies
"Enriched" Studies
Within the scope of enriched studies, the treatment data recorded in ADOReg can be expanded for specific studies
Database Studies
Retrospective analyses from the ADOReg database
The ADOReg in numbers
Patients in the ADOReg
10.974
Patients in the registry’s 5 entities
9.326
Malignant Melanoma
555
Basal Cell Carcinoma
794
Merkel Cell Carcinoma
404
Squamous Cell Carcinoma
206
Cutaneous Lymphoma
Study sites
67
Real world data are becoming increasingly important worldwide
Both in the USA and in Europe, efforts are being made to optimize the use of real world evidence and to increasingly use findings from clinical registers to inform health care research (see e.g. FDA, Framework for FDA’s Real-World Evidence Program, 2018). In the German healthcare system, real world evidence from registry data is also playing an increasingly important role, e.g. for use as a basis for the extended benefit assessment of drugs according to § 35 SGB (see IQWIG, „Rapid Report A19-43“, 10.01.2020).
Cooperation with the ADOReg and its established network of experts is ideally suited for the collection and evaluation of data in dermatological oncology
The requirements of the Institute for Quality and Efficiency in Health Care (IQWIG) regarding the quality of treatment data in clinical registers in Germany are implemented in the ADOReg. By setting the highest standards for the quality of the recorded treatment data, the ADOReg makes significant contributions to the care and contract research for a wide range of stakeholders in order to create a positive added value for all those involved in the healthcare system.
An online quality assurance and documentation system is the basis for the registry's program logic and database. Various measures ensure the high data quality in the ADOReg:
Self-Validation & Cross-Validation: To ensure high data quality, the system automatically checks the data entered by the documentarian, for example, whether it lies within a predefined value range (self-validation). It also identifies contradictory data entries (cross-validation).
Remote Monitoring & Queries: In addition to the automatic checking of data during input, the ADOReg database is regularly checked by monitors. These are clarified with the documentarians by telephone or, in campaigns, also on site.
On-Site Monitoring & Source Data Verification: To ensure the quality of the data, project-specific on-site monitoring can be carried out in the participating sites.
Training material, training on data collection and data entry: The registry is well described and there is a manual for data entry. In addition, the documentarians in the sites are invited at least once a year to attend a two-day training session.
One of the most important goals of the registry is to track patients over a long period of time. This means that data on a patient is entered several times at different time points in different treatment contexts (e.g. therapy cycles, follow-up care or secondary diseases) and, with a high probability also by different treatment institutions (e.g. outpatient, inpatient, change of physician).
These requirements and the need for longitudinal and cross-institutional analyses form the basis that data protection measures must meet. To ensure compliance with data protection in accordance with the General Data Protection Regulation within the framework of the ADOReg, several complementary technical, contractual and organisational measures are taken.
From a technical point of view, the separation of data entry, pseudonymisation and data storage is crucial for data protection. The central element of data protection is the Master-Patient-Index (MPI) according to a recognised procedure. The MPI enables the strict separation of personal data from medical data as well as a secure pseudonymisation of medical data. The security of the pseudonym is guaranteed by an independent third party. The independence of this body results from the technical and organizational separation of the ADOReg and the underlying contracts (especially with regards to the independence from instructions).
Publications
Our goal is to publish the studies conducted in cooperation with the ADOReg in a peer-reviewed journal. If you have any questions regarding the publication process, please contact the Academic Project Advisory Board or the Steering Group (see contact persons) of the ADOReg directly.
Moritz R. K. C., Gutzmer R., Zimmer L. et al. (2021) SARS-CoV-2 infections in melanoma patients treated with PD-1 inhibitors: A survey of the German ADOREG melanoma registry. European Journal of Cancer. 2021 Feb; 144:382-385. doi:10.1016/j.ejca.2020.11.015
Background:
Skin cancers are known for their strong immunogenicity, which may contribute to a high treatment efficacy of immune checkpoint inhibition (ICI). However, a considerable proportion of patients with skin cancer is immuno-compromised by concomitant diseases. Due to their previous exclusion from clinical trials, the ICI treatment efficacy is poorly investigated in these patients. The present study analyzed the ICI treatment outcome in advanced patients with skin cancer with a concomitant hematological malignancy.
Methods:
This retrospective multicenter study included patients who were treated with ICI for locally advanced or metastatic melanoma (MM), cutaneous squamous cell carcinoma (cSCC), or Merkel cell carcinoma (MCC), and had a previous diagnosis of a hematological malignancy irrespective of disease activity or need of therapy at ICI treatment start. Comparator patient cohorts without concomitant hematological malignancy were extracted from the prospective multicenter skin cancer registry ADOREG. Treatment outcome was measured as best overall response, progression-free (PFS), and overall survival (OS).
Results:
84 patients (MM, n=52; cSCC, n=15; MCC, n=17) with concomitant hematological malignancy were identified at 20 skin cancer centers. The most frequent concomitant hematological malignancies were non-Hodgkin’s lymphoma (n=70), with chronic lymphocytic leukemia (n=32) being the largest entity. While 9 patients received ICI in an adjuvant setting, 75 patients were treated for advanced non-resectable disease (55 anti-PD- 1; 8 anti-PD-L1; 5 anti-CTLA-4; 7 combinations). In the latter 75 patients, best objective response (complete response+partial response) was 28.0%, disease stabilization was 25.3%, and 38.6% showed progressive disease (PD). Subdivided by skin cancer entity, best objective response was 31.1% (MM), 26.7% (cSCC), and 18.8% (MCC). Median PFS was 8.4 months (MM), 4.0 months (cSCC), and 5.7 months (MCC). 1-year OS rates were 78.4% (MM), 65.8% (cSCC), and 47.4% (MCC). Comparison with respective ADOREG patient cohorts without hematological malignancy (n=392) revealed no relevant differences in ICI therapy outcome for MM and MCC, but a significantly reduced PFS for cSCC (p=0.002).
Conclusions:
ICI therapy showed efficacy in advanced patients with skin cancer with a concomitant hematological malignancy. Compared with patients without hematological malignancy, the observed ICI therapy outcome was impaired in cSCC, but not in MM or MCC patients.
Leiter U., Loquai C., Reinhardt L. et al. (2020) Immune checkpoint inhibition therapy for advanced skin cancer in patients with concomitant hematological malignancy: a retrospective multicenter DeCOG study of 84 patients. Journal for ImmunoTherapy of Cancer 2020;8:e000897. doi:10.1136/jitc-2020-000897
Background:
GNAQ and GNA11 mutant non‐uveal melanoma represent a poorly characterized, rare subgroup of melanoma with a gene mutation profile similar to uveal melanoma.
Objectives:
To characterize these tumours in terms of clinical behaviour and genetic characteristics.
Methods:
Patients with non‐uveal GNAQ/11 mutated melanoma were identified from the prospective multicentre tumour tissue registry ADOREG, Tissue Registry in Melanoma (TRIM) and additional cooperating skin cancer centres. Extensive data on patient, tumour and treatment characteristics were collected retrospectively. Targeted sequencing was used to determine tumour mutational burden. Immunohistochemistry staining was done for PD‐L1 and BAP1. Existing whole‐exome cutaneous and uveal melanoma data was analysed for mutation type and burden.
Results:
We identified 18 patients with metastatic GNAQ/11 mutant non‐uveal melanoma. Tumours had a lower tumour mutational burden and less UV‐signature mutations than cutaneous melanomas. In addition to GNAQ and GNA11 mutations (9 each), 6 SF3B1, 3 EIF1AX and 4 BAP1 mutations were detected. In contrast to uveal melanoma, GNAQ/11 mutant non‐uveal melanomas frequently metastasized lymphatically and concurrent EIF1AX, SF3B1 and BAP1 mutations were not apparently associated with patient prognosis. Objective response to immunotherapy was poor with only one partial response observed in ten treated patients (10%).
Conclusions:
Our findings suggest that GNAQ/11 mutant non‐uveal melanomas are a melanoma subtype distinct both clinically and genetically from cutaneous and uveal melanoma. As they respond poorly to available treatment regimens, novel effective therapeutic approaches for affected patients are urgently needed.
Livingstone E., Zaremba A., Horn S. et al. (2020) GNAQ and GNA11 Mutant Non‐Uveal Melanoma, a Subtype Distinct From Both Cutaneous and Uveal Melanoma. British Journal of Dermatology. DOI:10.1111/bjd.18947
Background:
Programmed cell death protein 1 (PD-1) checkpoint inhibition has recently advanced to one of the most effective treatment strategies in melanoma. Nevertheless, a considerable proportion of patients show upfront therapy resistance and baseline predictive biomarkers of treatment outcome are scarce. In this study we quantified PD-1 and programmed death-ligand 1 (PD-L1) in baseline sera from melanoma patients in relation to therapy response and survival.
Patients and methods:
Sera taken at therapy baseline from a total of 222 metastatic melanoma patients (two retrospectively selected monocentric discovery cohorts, n ¼ 130; one prospectively collected multicentric validation cohort, n ¼ 92) and from 38 healthy controls were analyzed for PD-1 and PD-L1 concentration by sandwich enzyme-linked immunosorbent assay.
Results:
Melanoma patients showed higher serum concentrations of PD-1 (P ¼ 0.0054) and PD-L1 (P < 0.0001) than healthy controls. Elevated serum PD-1 and PD-L1 levels at treatment baseline were associated with an impaired best overall response (BOR) to anti-PD-1 (P ¼ 0.014, P ¼ 0.041), but not to BRAF inhibition therapy. Baseline PD-1 and PD-L1 serum levels correlated with progression-free (PFS; P ¼ 0.0081, P ¼ 0.053) and overall survival (OS; P ¼ 0.055, P ¼ 0.0062) in patients who received anti-PD-1 therapy, but not in patients treated with BRAF inhibitors. By combining both markers, we obtained a strong discrimination between favorable and poor outcome of antiPD-1 therapy, with elevated baseline serum levels of PD-1 and/or PD-L1 associated with an impaired BOR (P ¼ 0.037), PFS (P ¼ 0.048), and OS (P ¼ 0.0098). This PD-1/PD-L1 combination serum biomarker was confirmed in an independent multicenter validation set of serum samples prospectively collected at baseline of PD-1 inhibition (BOR, P ¼ 0.019; PFS, P ¼ 0.038; OS, P ¼ 0.022). Multivariable Cox regression demonstrated serum PD-1/PD-L1 as an independent predictor of PFS (P ¼ 0.010) and OS (P ¼ 0.003) in patients treated with PD-1 inhibitors.
Conclusion:
Our findings indicate PD-1 and PD-L1 as useful serum biomarkers to predict the outcome of PD-1 inhibition therapy in melanoma patients and to select patients for PD-1-based versus BRAF-based therapy strategies.
Ugurel S., Schadendorf D., Horny K. et al. (2020) Elevated baseline serum PD-1 or PD-L1 predicts poor outcome of PD-1 inhibition therapy in metastatic melanoma. Annals of Oncology. 2020;31(1):144-152. DOI: 10.1016/j.annonc.2019.09.005
https://www.sciencedirect.com/science/article/pii/S0923753419354067
Background:
- Nivolumab monotherapy (NIVO) and nivolumab plus ipilimumab combination therapy (NIVO+IPI) are approved for the treatment of advanced melanoma in first and subsequent lines of therapy. In addition, NIVO is indicated for the adjuvant treatment of melanoma after complete resection. 1
- Real-world data complement available clinical data on the effectiveness and tolerability of NIVO / NIVO+IPI in a broader patient population.
- NICO (NCT02990611) is an ongoing prospective, multicenter non-interventional study (NIS) in Germany associated to ADOReg.
- NICO collects real-world data from patients treated with NIVO and NIVO+IPI, respectively, diagnosed with advanced/metastatic melanoma as well as from patients with adjuvant NIVO therapy after complete surgical resection.2
Study Design:
Population: In total, 950 adult patients treated according to marketing authorization in Germany, will be enrolled in three cohorts:
- Cohort 1+2 (n=750): patients with advanced melanoma, who start therapy with NIVO+IPI or NIVO
- Cohort 3 (n=200): patients treated with NIVO as adjuvant therapy
- Primary objectives: overall survival (OS) in cohort 1 (NIVO+IPI); relapse-free survival (RFS) in cohort 3 (adjuvant NIVO)
- Secondary objectives include progression free survival (PFS), response rates, safety profile, and quality of life (QoL)
Results:
Cohort 1 & 2: Baseline Characteristics
238 patients in cohort 1 (60.1%) and 159 patients in cohort 2 (70.0%) received NIVO+IPI and NIVO as first line (1L) therapy. Demographic parameters of 1L patients reflect a population similar to that of the pivotal study CheckMate 067 (CM 067)3 in regard to parameters such as age, sex and ECOG performance status.
Cohort 1 & 2: Overall Survival
Overall survival of 1L patients in NICO at 12 months is 70.3% (95% CI 62.8–76.6%) with NIVO+IPI and 72.0% (95% CI 62.4–79.5%) with NIVO. Observed OS in NICO is comparable to that observed in the pivotal CM 067 trial (72.3% and 73.1%, respectively).4
Cohort 1 & 2: Adverse Events
More side effects were reported for NIVO+IPI combination therapy (cohort 1) than for NIVO monotherapy (cohort 2). In line with these findings, more treatment discontinuations (66 [16.7%] vs 18 [7.9%]) and more treatment interruptions (117 [29.5%] vs. 28 [12.3%]) due to TrAE of any grade occurred with NIVO+IPI than with NIVO.
In total, 3 grade 5 TrAE occurred with NIVO+IPI; 1 case was due to autoimmune hepatitis and 2 were due to general disorders. With NIVO, 2 grade 5 TrAE occurred; 1 case was due to autoimmune disorder (multi organ failure) and 1 was due to general disorders.
Summary and Conclusion:
Presented data from the current interim analysis of NICO cohort 1 and 2 show 12 months effectiveness data for advanced melanoma patients treated with NIVO+IPI or NIVO in routine care in Germany.
- With the exception of a distinctively higher prevalence of brain metastases and more patients with LDH > ULN in the NICO study, patient characteristics are generally comparable to those of the corresponding pivotal clinical trial CM 067.
- 12-month OS in NICO was comparable to OS observed in the CM 067 trial even though some patient characteristics show less favorable health conditions in NICO.
- Treatments were generally well tolerated based on safety data from 623 patients. Data confirmed the safety profiles for NIVO and NIVO+IPI as determined in clinical trials.
References:
1. Summary of product characteristics OPDIVO® (01/2020) and YERVOY®(01/2020)
- ClinicalTrials.gov Identifier: NCT02990611; BMS study number: CA209-654
- Larkin et al. N Engl J Med 2015; 373:23
- Larkin et al. N Engl J Med 2019; 381:1535
Bei Fragen zur Originalpublikation wenden Sie sich bitte an Bristol Myers Squibb:
https://www.bmsmedinfo.de/emirf_de
Telefon: 0800 0752002
www.bmsmedinfo.de
Schadendorf D., Eigentler T., Mohr P., et al. (2020) NICO: Real world evidence in advanced melanoma; a national prospective non-interventional study of nivolumab monotherapy or in combination with ipilimumab in patients with advanced melanoma and in patients with adjuvant nivolumab therapy. Poster presented at the 34th Annual German Cancer Congress 2020
Einleitung:
- Wirksamkeit und Sicherheit von Nivolumab (NIVO) als Monotherapie oder in Kombination mit Ipilimumab (NIVO + IPI) bei der Behandlung des fortgeschrittenen Melanoms wurden in mehreren zulassungsrelevanten Studien für die dort definierten Patientenkollektive gezeigt. 1–7
- Die multizentrische, prospektive, mit dem ADOREG assoziierte, nicht-interventionelle Studie NICO (NCT02990611) erhebt Real-World-Daten bei Patienten mit fortgeschrittenem Melanom in Deutschland, bei denen eine Therapie mit NIVO + IPI oder NIVO innerhalb der zugelassenen Indikation durchgeführt wird.8,9
Studiendesign:
Population: Insgesamt 1050 volljährige Patienten (Kohorte 1 und 2: 750 Patienten, Kohorte 3: 300 Patienten), die eine Behandlung mit NIVO + IPI oder mit NIVO entsprechend der zugelassenen Indikation beginnen, werden in drei Kohorten der Studie eingeschlossen und über fünf Jahre dokumentiert:
- Kohorte 1 (K1): Patienten mit metastasiertem, nicht-resezierbarem Melanom; Behandlung mit NIVO + IPI
- Kohorte 2 (K2): Patienten mit metastasiertem, nicht-resezierbarem Melanom; Behandlung mit NIVO
- Kohorte 3 (K3): Patienten nach kompletter Tumorresektion; adjuvante Behandlung mit NIVO (Daten dieser Kohorte hier nicht vorgestellt)
- Primäre Endpunkte: Gesamtüberleben (OS) in K1; rezidivfreies Überleben (RFS) in K3
- Sekundäre Endpunkte: u. a. OS in K2, progressionsfreies Überleben (PFS), Ansprechraten, Sicherheitsprofil und Lebensqualität (QoL)
Ergebnisse der Kohorten 1 und 2:
Patientencharakteristika
Zum Zeitpunkt der dritten Interimsanalyse (Mai 2020) waren insgesamt 767 Patienten mit metastasiertem, nicht-resezierbarem Melanom in die Studie eingeschlossen, davon 486 in K1 (NIVO + IPI) und 281 in K2 (NIVO). In K1 waren 60,3 % und in K2 63,7 % der Patienten männlich. Die Patienten in K1 waren im Vergleich zur K2 jünger (durchschnittliches Alter 61 versus 69 Jahre); ein größerer Anteil der Patienten in K1 wies bei Studieneinschluss Hirnmetastasen auf (33,5 % versus 18,5 %) und hatte einen erhöhten LDH-Wert (44,4 % versus 39,9 %)
Im Vergleich zu den zulassungsrelevanten Studien wurden in Rahmen von NICO unter anderem mehr Patienten in späteren Behandlungslinien, mehr ältere Patienten und mehr Patienten mit schlechterem ECOG-Performance Status, mit erhöhtem LDH-Wert oder mit Hirnmetastasen behandelt.
Gesamtüberleben (OS) in Kohorte 1 (NIVO + IPI)
Unter Kombinationstherapie mit NIVO + IPI (K1) in der Erstlinie (1 L) lag das Gesamtüberleben (OS) in NICO nach 24 Monaten bei 50,8 % (95 % KI 41,3 — 59,5 %). Das geschätzte mediane Gesamtüberleben (mOS) unter NIVO + IPI in der 1 L lag bei 25,9 Monaten (95 % KI 19,5 — nicht ermittelbar [n. e.]).
In späteren Behandlungslinien (≥ 2 L) unter NIVO + IPI lag das OS nach 24 Monaten in NICO bei 36,7 % (27,6 — 45,8 %). Das geschätzte mOS unter NIVO + IPI in ≥ 2 L lag bei 11,7 Monaten (95 % KI 7,5 — 16,7).
Gesamtüberleben (OS) in Kohorte 2 (NIVO)
Unter Monotherapie mit NIVO (K2) in der Erstlinie (1 L) lag das OS nach 24 Monaten in NICO bei 48,6 % (95 % KI 36,0 — 60,1 %). Das geschätzte mOS unter NIVO in der 1 L lag bei 22,0 Monaten (15,4 — n. e.).
In späteren Behandlungslinien (≥ 2 L) unter NIVO lag das OS nach 24 Monaten in NICO bei 46,0 % (31,1 — 59,8 %). Das geschätzte mOS unter NIVO in ≥ 2 L lag bei 18,3 Monaten (11,1 — n. e.).
Ansprechraten
Unter Kombinationstherapie mit NIVO + IPI (K1) lag die beste Gesamtansprechrate (BORR) in der Erstlinie (1 L) bei 54,2 % und in späteren Behandlungslinien (≥ 2 L) bei 34,7 %.
Unter Monotherapie mit NIVO (K2) lag die BORR in der 1 L bei 48,5 % und in ≥ 2 L bei 40,4 %.
Sicherheit
Unter Kombinationstherapie mit NIVO + IPI (K1) wurden mehr behandlungsbedingte unerwünschte Ereignisse (treatment-related adverse events, TrAEs) Grad 3 und 4 beobachtet als unter Monotherapie mit NIVO (K2). Die insgesamt häufigsten TrAEs Grad 3 und 4 waren Kolitis (7,2 %) und Autoimmunerkrankungen (5,6 %).
In K1 kam es bei 5,1 % der Patienten zu Behandlungsabbrüchen und bei 15,4 % zu Behandlungsunterbrechungen aufgrund TrAEs. In K2 kam es bei 1,4 % zu Behandlungsabbrüchen und bei 7,5 % zu Behandlungsunterbrechungen aufgrund TrAEs.
In K1 wurden insgesamt fünf TrAEs Grad 5 beobachtet (je ein Fall von Autoimmunerkrankung, Störung des Blut- oder Lymphsystems, Kolitis, Neoplasie und Tod sonstiger Ursache). In K2 wurden insgesamt drei TrAEs Grad 5 beobachtet (je ein Fall von Autoimmunerkrankung, Kolitis und Pneumonie).
Zusammenfassung:
- Die Ergebnisse der dritten Interimsanalyse der nicht-interventionellen Studie NICO umfassen aktuelle Real-World-Daten zur Anwendung von Nivolumab in Kombination mit Ipilimumab oder als Nivolumab Monotherapie bei Patienten mit metastasiertem, nicht-resezierbaren Melanom in Deutschland.
- Die Daten zeigen, dass im klinischen Alltag auch ältere Patienten sowie Patienten mit ECOG-Performance Status ≥ 2, mit erhöhtem LDH-Wert und mit Hirnmetastasen behandelt werden.
- Zwischen den Kohorten K1 (NIVO + IPI) und K2 (NIVO) sind Unterschiede in Bezug auf Patientencharakteristika und prognostische Faktoren zu beobachten.
- Das Gesamtüberleben (OS) nach 24 Monaten deutet auf eine gute Wirksamkeit von NIVO + IPI und NIVO auch bei Patienten mit schlechterem Risikoprofil hin, sowohl in der Erstlinie als auch in späteren Behandlungslinien.
- Im Vergleich z. B. zur CheckMate 067-Studie1,2 wurden in NICO auch Patienten mit ungünstigen prognostischen Faktoren (wie ungünstiger ECOG, erhöhte LDH, oder Hirnmetastasen) eingeschlossen, durch die sich ggf. Unterschiede im klinischen Outcome erklären.
- Die Daten bestätigen die in klinischen Studien gezeigten Sicherheitsprofile von NIVO + IPI und NIVO. Das in der klinischen Routine berichtete Sicherheitsprofil stimmt mit dem in anderen Studien beobachteten Sicherheitsprofil dieser Medikamente überein.
Referenzen:
- Larkin et al. N Engl J Med 2015;373:23-34
- Larkin et al. N Engl J Med 2019; 381:1535-46
- Robert et al. N Engl J Med 2015;372:320-30.
- Ascierto et al. JAMA Oncol 2018; 5:187
- Weber et al. Lancet Oncol 2015; 16:375
- Hodi et al. N Engl J Med 2015; 372:2006-17
- Hodi et al. Lancet Oncol 2016; 17:1558-68
- ClinicalTrials.gov Identifier: NCT02990611; BMS study number: CA209-654
- Aktuelle Fachinformationen OPDIVO® (Nivolumab) und YERVOY® (Ipilimumab)
Bei Fragen zur Originalpublikation wenden Sie sich bitte an Bristol Myers Squibb:
https://www.bmsmedinfo.de/emirf_de
Telefon: 0800 0752002
www.bmsmedinfo.de
Ugurel S., Satzger I., Eigentler T. et al. (2020) Real-World-Daten zur Anwendung von Nivolumab als Monotherapie oder in Kombination mit Ipilimumab in der Behandlung des fortgeschrittenen Melanoms: Zwischenergebnisse der nicht-interventionellen Studie NICO. Poster präsentiert auf dem 30. Deutschen Hautkrebskongress 2020
Background:
Melanoma brain metastases (MBMs) are common among patients with advanced/metastatic disease
- 28.2% of patients have been estimated to have MBMs at diagnosis, and 44% were found to develop MBMs during the course of treatment1,2
- The prognosis of patients with MBMs has historically been poor, with median overall survival (OS) estimated at 4.7 months in one study and MBMs frequently reported to contribute to death2
- These patients are often excluded from prospective clinical trials because of their poor prognosis
- Comparatively little is known about the effectiveness and tolerability of standard treatment options for metastatic melanoma, such as nivolumab (NIVO) alone or in combination with ipilimumab (NIVO+IPI), in this vulnerable patient population
- NIVO+IPI treatment was associated with an intracranial objective response rate (ORR) of 54% and a median OS that had not been reached (at a follow up of 20.6 months) in one study (CheckMate 204) 3 and a median OS of 32.8 months in another study (ABC trial)4
- Treatment with NIVO monotherapy was associated with an intracranial ORR of 20% and a median OS of 26.1 months in the ABC trial4
- Real world evidence concerning the use of these agents in the first and later line settings is limited
- The current study evaluated real world treatment patterns, effectiveness, and safety in patients with MBMs treated with NIVO+IPI or NIVO monotherapy
Results:
Patients and treatment patterns:
- A total of 767 patients with advanced melanoma were enrolled, of whom 215 (28%) had MBMs
- Most patients with MBMs were male (64%); 10% had an ECOG performance status (PS) ≥ 2
- Among patients with MBMs, a higher proportion of those treated with NIVO+IPI versus NIVO had received prior
radiotherapy (29% vs 19%) and/or surgery (18% vs 6%)
- Among all patients in the study, approximately 2/3 received NIVO+IPI and 1/3 received NIVO
- In patients with MBMs, approximately 3/4 received NIVO+IPI and 1/4 received NIVO
- A higher proportion of patients aged ≤ 65 years were treated with NIVO+IPI (60%) than with NIVO (34%)
- In contrast, higher proportions of patients aged > 65 or > 75 years received NIVO than NIVO+IPI (65% vs 37% and 37% vs 15%, respectively)
Efficacy:
- In patients with MBMs, ORRs were higher with NIVO+IPI (26%) than with NIVO (15%)
- ORRs were approximately 10 percentage points higher with the combination than with monotherapy,
irrespective of the setting (1L: 30% vs 19%, 2L+: 20% vs 8%)
- Notably, the combination was administered more frequently as 2L+ treatment than was NIVO alone in patients with MBMs
- In the overall population, ORRs with NIVO+IPI versus NIVO were 54% versus 48% in the 1L setting and 35% versus 40% in the 2L+ settings, respectively
- Disease control rates (DCRs) were similar in both treatment groups, reflecting a higher percentage of patients with objective response in the NIVO+IPI group and stable disease (SD) in the NIVO group
- The presence of MBMs was associated with a poor prognosis with both NIVO+IPI and NIVO, which became even worse in patients with MBMs who received 2L+ versus 1L therapy (e.g. median OS with NIVO+IPI: 9.9 vs 23.5 months)
- The number of MBMs appeared to have comparatively little effect on OS in patients treated with NIVO+IPI or NIVO (although numbers of patients were small)
- Among patients with MBMs, those with a BOR of CR or PR derived a high level of OS benefit with both treatments, in contrast to patients with a BOR of SD or PD
- Preliminary results indicated that treatment with NIVO+IPI or NIVO was effective in patients with BRAF mutant and wild type tumors (data not shown), consistent with results reported in clinical trials
Safety:
- Grade 3−4 treatment related adverse events (TRAEs) were reported in 41% of all patients and 27% of patients with MBMs treated with 1L NIVO+IPI
- Grade 3−4 TRAEs were reported in 19% of all patients and 26% of those with MBMs treated with 1L NIVO
- A total of 8 patients died due to TRAEs: 5 in the NIVO+IPI group and 3 in the NIVO group
Conclusion:
- In this real world study, both NIVO+IPI and NIVO provided effective and generally tolerable treatment options for patients with advanced melanoma
- NIVO+IPI was preferred to NIVO as the treatment of choice, particularly among younger patients and those with MBMs
- ORR was approximately 10 percentage points higher in patients with MBMs treated with NIVO+IPI than with NIVO
- Real world patients with MBMs showed OS benefit with NIVO+IPI or NIVO in the 1L as well as in the 2L+ setting
- OS results with 1L NIVO+IPI were comparable to those of the ABC trial, but did not reach the high OS levels observed with the more highly selected patient population in CheckMate 204 (1 year OS: NICO, 59%; ABC, 63%; CheckMate 204, 82%)3,4
- OS benefit was pronounced in patients with MBMs who achieved a CR or PR, suggesting that achieving a deep response is a particularly important goal
- TRAE incidences in this real word study were largely consistent with prior clinical trials3
- Further analyses are needed to compare the safety profile of patients with MBMs with that of the total population
- Limitations of these results include their descriptive nature, imbalances between the treatment groups, and the immaturity of the current data
References:
- 1. Cagney DN, et al. Neuro Oncol 2017;19:1511−1521.
- Davies MA, et al. Cancer 2011;117:1687−1696.
- Tawbi H, et al. Oral presentation at ASCO 2019; May 31−June 4, 2019; Chicago, IL. Presentation 9501.
- Long GV, et al. Poster presentation at ESMO 2019; September 27−October 1, 2019; Barcelona, Spain. Presentation 1311O.
Bei Fragen zur Originalpublikation wenden Sie sich bitte an Bristol Myers Squibb:
https://www.bmsmedinfo.de/emirf_de
Telefon: 0800 0752002
www.bmsmedinfo.de
Gutzmer R., Eigentler T., Mohr P. et al. (2020) Nivolumab monotherapy or combination therapy with ipilimumab in advanced melanoma patients with brain metastases: real world evidence from the German non interventional study NICO. Poster presented at the ESMO Virtual Congress 2020
Background:
Patient-reported outcomes regarding health-related quality of life (HRQoL) were assessed as secondary or exploratory outcomes in phase II/III clinical trials with nivolumab plus ipilimumab (NIVO+IPI) combination therapy and nivolumab (NIVO) monotherapy in advanced melanoma; NIVO and NIVO+IPI maintained HRQoL in these trials.1-4
NICO (NCT02990611)5 is a prospective, multicenter, non-interventional study in Germany associated to ADOReg, collecting real-world data on the use of NIVO+IPI or NIVO in patients with advanced melanoma as well as on the use in patients treated with adjuvant NIVO after complete surgical resection.
Objectives:
To describe longitudinal change from baseline in European Organization for Research and Treatment of Care (EORTC) Quality of Life Questionnaire (QLQ-C30) scores in NICO separately for advanced (unresectableor metastatic) melanoma patients treated with NIVO+IPI or NIVO overall and among patients treated as first line therapy (1L) or as a second or later line of therapy (≥ 2L).
Methods:
- HRQoL was assessed at baseline, week 6, month 3/6/9/12 using the European Organization for Research and Treatment of Care (EORTC) QLQ-C30 Questionnaire
- Analyses were performed on all advanced melanoma patients with baseline and ≥ 1 post-baseline assessment
- For an analysis at each visit separately, the mean within-person change from baseline were calculated for each visits; change in scores were tested using the paired t-test (P≤0.05)
- For an analysis taking the complete observation time into account, a mixed effects model for repeated measures (MMRM), with time point as fixed effect, study day as a random effect, time point as a repeated measure and baseline score as a covariate was performed
Results:
Questionnaire completion rate and patient characteristics
- 766 patients with advanced melanoma were treated with NIVO+IPI (487 patients) or NIVO (279 patients)
- EORTC QLC-C30 completion rate at baseline: 86% (656 patients)
- 470 patients with baseline and ≥1 post-baseline assessment were included in HRQoL analysis population
- NIVO+IPI: 298 patients (61%)
- NIVO: 172 patients (62%)
- HRQoL analysis population is similar to all advanced melanoma patients in NICO with some differences in ECOG performance status, LDH level, and presence of brain metastases
Subgroup Analysis - HRQoL in patients with adverse events
- The majority of adverse events occurred during the first 3 months of treatment
- Clinically meaningful transient decline in EORTC QLC-C30 at month 3 for NIVO+IPI population, and maintained HRQolafterwards
- Clinically meaningful improvement at month 12 and overall time period for NIVO population
Subgroup analysis based on response
- In general, stabilization of HRQoL in both patient cohorts
- Clinically meaningful improvement of HRQoL at 12 months for NIVO patients
Conclusion:
- Patients in NICO treated with NIVO+IPI or NIVO for advanced melanoma, maintained HRQoL, and for patients treated with NIVO overall and 1L, statistically significant and clinically meaningful improvement was shown
- Exploratory subgroup analysis have been carried out on patients experiencing adverse events grade 3 and 4 as well as patients with complete or partial response
- The rate of grade 3/4 adverse events was higher in NIVO+IPI patients than NIVO patients, with most events occurring within the first 3 months of treatment
- A clinically meaningful transient decline in HRQoL was observed at month 3 for NIVO+IPI patients who experienced grade 3/4 adverse events
- These data suggest that declines in HRQoL may be related to onset of adverse events, however the overall trend shows maintained HRQoL over time
- In patients with complete or partial response a stabilization of HRQoLwith both, NIVO+IPI and NIVO was observed
- HRQoL observed in this real-world study is in line with published data from clinical trials
References:
1. Long GV, et al. Poster presented at American Society of Clinical Oncology 2015 Annual Meeting; May 29–June 2, 2015; Chicago, IL, USA;
2. Abernethy AP, et al. Poster presented at American Society of Clinical Oncology Annual Meeting (ASCO); May 29–June 2, 2015; Chicago, IL, USA;
3. Schadendorf D, et al. Poster presented at American Society of Clinical Oncology Annual Meeting (ASCO); May 31-June 4, 2019; Chicago, IL, USA. Abstract 9551;
4. Schadendorf D, et al. Europ J Cancer2017;82:80-91;
5. Clinicaltrials.gov; https://clinicaltrials.gov/ct2/show/NCT02990611. EORTC, European Organization for Research and Treatment of Care.
Bei Fragen zur Originalpublikation wenden Sie sich bitte an Bristol Myers Squibb:
https://www.bmsmedinfo.de/emirf_de
Telefon: 0800 0752002
www.bmsmedinfo.de
Mohr P., Eigentler T., Gutzmer R. et al. (2020) Real-world outcomes on health-related quality of life in the non-interventional study NICO in Germany. Presentation at 16th EADO Congress 2020
Background:
- Advanced melanoma, in particular unresectable stage III and stage IV disease, is an aggressive tumor with a poor prognosis. There is limited understanding of real-world utilization patterns and outcomes associated with immunotherapies like the anti-programmed death 1 (PD-1) antibody pembrolizumab in advanced melanoma patients.
- Assessing the clinical benefit of cancer treatments in real-world settings is often hampered by lack of high-quality structured data for classical endpoints. In particular, objective remission measured by Response Evaluation Criteria in Solid Tumors (RECIST) and progression-free survival (PFS) status data derived from that have limitations in clinical practice.
- Time on treatment (ToT) and time to next treatment (TtNT) are composite endpoints measuring time from initiation to discontinuation of treatment and start of a subsequent treatment or death, respectively. They may potentially serve as an indicator for clinical tumor control and a surrogate marker for treatment benefit.
- In clinical tumor registration, ToT, TtNT, and overall survival (OS) are all robust and reliable endpoints and can also be reliably retrieved retrospectively from medical chart data.
- The ADOReg platform was developed in 2014 and collects data on melanoma patients treated at one of 50 certified skin cancer centers. ADOReg captures approximately one-third to one-half of all metastatic patients in Germany, and participating member hospitals and practices are distributed evenly within Germany. Physicians in these centers have unrestricted access to PD-1 inhibitors and targeted therapies.
Methods:
Study Design and Data Source:
Retrospective database study of real-world pembrolizumab utilization captured in the ADOReg registry.
Information on demographics, clinical history, treatment episode details, and clinical outcomes is collected utilizing standard electronic case report forms covering the whole disease and treatment history in routine practice
Study Population:
Inclusion Criteria:
- Advanced melanoma (unresectable stage III or stage IV) diagnosis at pembrolizumab initiation
Initiated pembrolizumab between June 1, 2014, and December 31, 2017
- Received at least 1 dose of pembrolizumab
- Aged 18 years or older on start date of pembrolizumab (ie, index date)
Exclusion Criteria:
– Received pembrolizumab in a clinical trial
– Treated with pembrolizumab for an indication other than advanced melanoma
Statistical Analysis:
- Demographic and baseline variables were calculated and displayed according to standard methods
- Survival parameters were calculated by Kaplan-Meier method using index date as the baseline date and appropriate definitions of event time points, ie, end of treatment for rwToT, clinical or radiologic progression for rwPFS, start of subsequent treatment or death for rwTtNT, and death due to any reason for rwOS
- Kaplan-Meier analyses were stratified for line of treatment, origin of primary, and presence of brain metastasis
- The correlation between rwToT, rwPFS, rwTtNT, and rwOS was analyzed using nonparametric rank correlation
- Database cutoff: June 20, 2019
Results:
- At the time of pembrolizumab initiation, the majority of patients had stage IV disease (Table 1). Of 664 patients, 402 were treatment-naïve and 262 were pretreated
- Most of the 664 patients were of known cutaneous origin (80.7%), while 4.5% were of ocular origin, 2.6% were of mucosal origin, and 12.2% had melanoma of unknown primary
- In first line, the median rwTtNT was markedly longer than in second line (11 mo vs 9.1 mo). However, in third line or higher, the median rwTtNT was 11.7 months. This was also reflected by 1-year next-treatment rates of 49.2%, 43.3%, and 49.5% in first, second, and third line plus, respectively
- Median rwTtNT was longest for unknown primary and cutaneous melanoma but markedly shorter for ocular and mucosal melanoma. In contrast, the presence vs absence of brain metastasis did not significantly affect median rwTtNT
- Out of the different outcome parameters, rwTtNT correlated highest with rwOS, while rwPFS showed the lowest correlation and rwToT was in between
Conclusions:
- In comparison to rwPFS, rwTtNT appears to be of higher relevance and discriminatory power and may be a valuable endpoint in real-world populations receiving immunotherapy
- Patients with brain metastasis show a comparable rwTtNT with pembrolizumab to the overall population
- For patients in higher line of treatment, rwTtNT suggests that treatment with pembrolizumab is likely to be a valuable option
- Data for patients with ocular or mucosal melanoma still reflects the more aggressive course in these subpopulations
Mohr P., Chandwani S., Leiter U. et al. (2019) Real-World Time to Next Treatment in Advanced Melanoma Patients Treated With Pembrolizumab in the German ADOReg Melanoma Registry. Poster presented at SMR Congress 2019
Background:
In melanoma, potential benefits of therapies after PD-1 inhibitor failure, including those BRAF positive patients who have already received combined BRAF-/MEK inhibitors before anti PD-1 are poorly defined. We therefore analyzed the treatment patterns and outcome of systemic therapies for patients after anti-PD-1 failure.
Methods:
From the ADOReg registry, patients fulfilling the following inclusion criteria were consecutively included until a number of 200 cases was reached. 1) Ipilimumab naive patients with unresectable metastatic cutaneous or mucosal melanoma. 2) Failure from treatment with a single agent anti PD-1 antibody. 3) Known BRAF status and, in case of BRAF-V600 mutation, BRAF-/MEK-inhibitor treatment prior to anti PD-1 treatment. 4) Consecutive systemic treatment started within a maximum of 6 months after anti PD-1 failure.
Objectives:
Rate of objective remissions (ORR), disease control (DCR), survival (OS), tolerability and disease patterns correlated to the use of different treatments after PD-1 treatment failure in real-life conditions in Germany. Results: In total 23.5 % of the patients received ipilimumab single agent, 38.5 % received the combination of ipilimumab and nivolumab (Ipi/Nivo), and the remaining various regimens. (Table) Ipi/Nivo resulted in an ORR significantly higher than for Ipi alone (p=0.02). In 18 patients receiving BRAF-/MEK inhibitor re-challenge, ORR was comparable to Ipi/Nivo. Conventional Chemotherapy was still in frequent use (dacarbazine n =33; other n=17), but response rates were low (ORR 6%). Some remission were also achieved by use of talimogene laherparepvec (n=2 out of 4).
Conclusions:
Treatment patterns of patients after anti-PD-1 failure differ remarkably. Although lower than reported in treatment naive patients, the combination of Ipilimumab and Nivolumab appeared favorable as compared to all other regimens, except for BRAF-/MEK inhibitor re-challenge which produced similar remission rates. Still, chemotherapies including dacarbazine are in clinical practice, though giving only poor outcome.
Weichenthal M., Ugurel S., Leiter U. et al. (2019) Salvage therapy after failure from anti-PD-1 single agent treatment: A Study by the German ADOReg melanoma registry. Journal of Clinical Oncology. 2019;37(15 suppl.). DOI: 10.1200/JCO.2019.37.15_suppl.9505. Poster presented at ASCO 2019
https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.15_suppl.9505
Background:
- Advanced melanoma, in particular unresectable stage III and stage IV disease, is an aggressive tumor with a poor prognosis. There is limited understanding of real-world utilization patterns and outcomes associated with immunotherapies like the anti-programmed death 1 (PD-1) antibody pembrolizumab in advanced melanoma patients.
- Assessing the clinical benefit of cancer treatments in real-world settings is often hampered by lack of high-quality structured data for classical endpoints. In particular, objective remission measured by Response Evaluation Criteria in Solid Tumors (RECIST) and progression-free survival (PFS) status data derived from that have limitations in clinical practice.
- Time on treatment (ToT) and time to next treatment (TtNT) are composite endpoints measuring time from initiation to discontinuation of treatment and start of a subsequent treatment or death, respectively. They may potentially serve as an indicator for clinical tumor control and a surrogate marker for treatment benefit.
- In clinical tumor registration, ToT, TtNT, and overall survival (OS) are all robust and reliable endpoints and can also be reliably retrieved retrospectively from medical chart data.
- The ADOReg platform was developed in 2014 and collects data on melanoma patients treated at one of 50 certified skin cancer centers. ADOReg captures approximately one-third to one-half of all metastatic patients in Germany, and participating member hospitals and practices are distributed evenly within Germany. Physicians in these centers have unrestricted access to PD-1 inhibitors and targeted therapies.
Methods:
Study Design and Data Source:
Retrospective database study of real-world pembrolizumab utilization captured in the ADOReg registry.
Information on demographics, clinical history, treatment episode details, and clinical outcomes is collected utilizing standard electronic case report forms covering the whole disease and treatment history in routine practice
Study Population:
Inclusion Criteria:
- Advanced melanoma (unresectable stage III or stage IV) diagnosis at pembrolizumab initiation
Initiated pembrolizumab between June 1, 2014, and December 31, 2017
- Received at least 1 dose of pembrolizumab
- Aged 18 years or older on start date of pembrolizumab (ie, index date)
Exclusion Criteria:
– Received pembrolizumab in a clinical trial
– Treated with pembrolizumab for an indication other than advanced melanoma
Statistical Analysis:
- Demographic and baseline variables were calculated and displayed according to standard methods
- Survival parameters were calculated by Kaplan-Meier method using index date as the baseline date and appropriate definitions of event time points, ie, end of treatment for rwToT, clinical or radiologic progression for rwPFS, start of subsequent treatment or death for rwTtNT, and death due to any reason for rwOS
- Kaplan-Meier analyses were stratified for line of treatment, origin of primary, and presence of brain metastasis
- The correlation between rwToT, rwPFS, rwTtNT, and rwOS was analyzed using nonparametric rank correlation
- Database cutoff: June 20, 2019
Results:
- At the time of pembrolizumab initiation, the majority of patients had stage IV disease (Table 1). Of 664 patients, 402 were treatment-naïve and 262 were pretreated
- Most of the 664 patients were of known cutaneous origin (80.7%), while 4.5% were of ocular origin, 2.6% were of mucosal origin, and 12.2% had melanoma of unknown primary
- In first line, the median rwTtNT was markedly longer than in second line (11 mo vs 9.1 mo). However, in third line or higher, the median rwTtNT was 11.7 months. This was also reflected by 1-year next-treatment rates of 49.2%, 43.3%, and 49.5% in first, second, and third line plus, respectively
- Median rwTtNT was longest for unknown primary and cutaneous melanoma but markedly shorter for ocular and mucosal melanoma. In contrast, the presence vs absence of brain metastasis did not significantly affect median rwTtNT
- Out of the different outcome parameters, rwTtNT correlated highest with rwOS, while rwPFS showed the lowest correlation and rwToT was in between
Conclusions:
- In comparison to rwPFS, rwTtNT appears to be of higher relevance and discriminatory power and may be a valuable endpoint in real-world populations receiving immunotherapy
- Patients with brain metastasis show a comparable rwTtNT with pembrolizumab to the overall population
- For patients in higher line of treatment, rwTtNT suggests that treatment with pembrolizumab is likely to be a valuable option
- Data for patients with ocular or mucosal melanoma still reflects the more aggressive course in these subpopulations
Mohr P., Chandwani S., Leiter U. et al. (2019) Real-World Time to Next Treatment in Advanced Melanoma Patients Treated With Pembrolizumab in the German ADOReg Melanoma Registry. Poster presented at SMR Congress 2019
Immune checkpoint inhibition using antibodies against Programmed-Death-1 receptor functioning has created a whole new class of successful cancer therapies. Advanced melanoma is among the pivotal tumors demonstrating high rate of long-term survivors in a previously hard-to-treat condition. We evaluated real world time on treatment (rwToT) in a large cohort of pembrolizumab treated patients in Germany.
Patients initiating pembrolizumab from Aug 2015 to Dec 2017 for unresectable stage III or stage IV melanoma were examined from the German national skin cancer registry (ADOReg). rwToT was calculated as the interval between first and last dose of pembrolizumab. Kaplan-Meier estimates for median rwToT and 1-year ontreatment rate were generated.
In total we evaluated 665 pembrolizumab patients with non-resectable stage III (n=65) or stage IV (n=600) melanoma. Of these, 387 were treatment-naive and 278 were pretreated with chemotherapy, BRAF inhibitor, or CTLA-4 blockade. In addition to cutaneous melanoma (n= 539), the study population consisted of ocular (n=30), mucosal (n=16), and unknown (n=80) origin of melanoma. In first-line, the median rwToT was markedly longer than in second-line (209 days vs. 148 days). However, in third-line or higher the median rwToT was 190 days. This was also reflected by 1-year on-treatment rates of 34.4%, 28.9%, and 30.1% in first-, second-, and third-line plus, respectively. Median rwToT was longest for unknown primary (228 days) and cutaneous melanoma (203 days), but markedly shorter for ocular (84 days) and mucosal (106 days) melanoma. In contrast, the presence vs. absence of brain metastasis did not affect median rwToT (189 vs. 196 days).
In conclusion, rwToT with pembrolizumab was reasonably long, even in patients receiving the treatment in third or higher line. The presence of brain metastasis did not result in shorter rwToT.
Mohr P., Chandwani S., Leiter U. et al. (2018) Time on Treatment with Pembrolizumab in 665 Advanced Melanoma Patients in a Real World Setting: A Retrospective Analysis from ADOReg – the German DeCOGMelanoma Registry. Poster presented at SMR Congress 2018
Background:
Targeted therapies with BRAF plus MEK inhibitors (BRAFi; MEKi) represent the major treatment strategy for patients with BRAF-mutated metastatic melanoma (MM). Previous analyses suggested a correlation between programmed death-ligand 1 (PD-L1) expression in tumour tissues and the outcome of targeted therapies. This study investigated PD-L1 as a potential predictive biomarker of BRAFi-based targeted therapies in MM patients.
Patients and methods:
We analysed two independent cohorts of BRAF V600-mutated MM patients undergoing BRAFi-based therapies for PD-L1 expression in pre-treatment tumour tissues. The oligocentre cohort 1 included 83 patients whose tumour tissues were analysed retrospectively with the anti-PD-L1 antibody clone E1L3N. The multicentre cohort 2 included 58 patients whose tumour tissues were analysed prospectively within the framework of the "Registry of the Arbeitsgemeinschaft Dermatologische Onkologie" (ADOREG) and "Tissue Registry in Melanoma" (TRIM) project using the anti-PD-L1 antibody clone 28-8.
Results:
PD-L1 expression in pre-treatment tumour tissue did not correlate with response or survival to BRAFi-based therapies in both MM patient cohorts. This finding was not influenced by retrospective versus prospective immunohistochemistry analyses, oligocentre versus multicentre cohorts or the different anti-PD-L1 antibody clones used. In cohort 1, PD-L1 positivity was detected in tumour tissue of 41.0% and 18.1% of patients (cut-off 1% and 5%, respectively). In cohort 2, 58.6% and 39.7% of patients showed PD-L1 positivity (cut-off 1% and 5%, respectively).
Conclusion:
In two independent cohorts including a total of 141 MM patients, PD-L1 expression in tumour tissue did not correlate with the outcome of BRAFi-based treatment. Therefore, PD-L1 cannot be recommended for the use as a predictive biomarker of BRAFi-based therapy in BRAF V600-mutated MM.
Schaper-Gerhardt K., Okoye S., Herbst R. et al. (2018) PD-L1 Status Does Not Predict the Outcome of BRAF Inhibitor Therapy in Metastatic Melanoma. Eur J Cancer. 2018;88:67–76. DOI:10.1016/j.ejca.2017.10.026
Introduction & Objectives:
In the treatment of metastatic melanoma (MM), significant progress has been made from the introduction of immune checkpoint inhibitors,and MAP kinase inhibitors in BRAF V600 mutations. Efficacy of programmed death (PD)-1 inhibitors is well known from clinical trials for selected patient populations meeting their selection criteria. Potential benefits of PD-1 inhibitors are not well described in real-world populations that may differ from trial populations.
Materials & Methods:
Patients initiating pembrolizumab from Aug 2015 to Dec 2017 for non resectable stage III or stage IV melanoma from the German national skin cancer registry (ADOReg) were investigated. All patients with a minimum set of available data for tumor characteristics, metastatic pattern, real world treatment response (rwTR), and follow-up were included. Survival analysis was done by Kaplan-Meier and Cox regression methods.
Results:
We evaluated 665 pembrolizumab treated patients with advanced stage III (n=65) or stage IV (n=600) melanoma; of these 387 were treatment naïve. The mean age was 66.4 years; 60.8% were males; 30.5%/52.6%/16.8% had mutant/wild type/unknown BRAF status; and there were 81.1%/2.4%/4.5% with cutaneous/mucosal/ocular origin of melanoma, and 12.0% with unknown primary. At time of treatment, 141 patients had brain metastasis;54.1%/36.0%/9.9% with normal/elevated/missing LDH; and 35.5%/18.9%/4.1%/41.5% with 0/1/>1/Unknown ECOG performance status (PS). Median duration of follow-up was 18.6 months. Overall, 204 patients (30.7%) achieved rwTR. This rate was higher for first line treatment (32.6%; n=387) as compared to second line (24.3%; n=144). However, for patients reaching third line plus treatment with pembrolizumab, the response rate was 32.1% (n=134). The overall median real-world progression free survival (rwPFS) was 4.9 months (95% CI 3.8, 6.4) and 12-month rwPFS rate was 34.0% (95% CI 30.1, 37.9). In first line, the median rwPFS was 6.0 months and the 12-month rwPFS rate 34.7%. Median overall survival (OS) was 33.5 months (95% CI 29.1, N/A) and 12-month OS rate was 73.7 (95% CI 69.8, 77.2). In third line plus treatment, 12-month OS rate was still 70.0%, and an early estimate of 24-month OS rate was similar to first line patients with 60.3% vs. 59.4%. Accordingly, line of treatment was not significantly predictive for rwPFS or OS, while extracutaneous origin, presence of brain metastasis, poor PS (ECOG >1), and elevated LDH were significantly associated with poor rwPFS or OS.
Conclusions:
This real-world study comprised of a more heterogeneous population of MM patients treated with pembrolizumab than those included in clinical trials. Remarkably, the study showed a good long term control, even with a third or higher line of treatment supporting the effectiveness of pembrolizumab in real-world clinical practice. Expectedly, some patient populations still have a dismal prognosis even with the use of modern treatments.
Mohr P., Chandwani S., Ugurel S. et al. (2018) Real World Data on 665 Patients Treated with Pembrolizumab for Metastatic Melanoma: An ADOReg Study - the Registry of the German Skin Cancer Society. Presented at EADO Congress 2018
Die immunonkologische Therapie mit anti‐PD1 Antikörpern (Nivolumab, Pembrolizumab) ist ein etabliertes Konzept in der Behandlung des metastasierten Melanoms. Trotzdem sind prädiktive Biomarker, die den Erfolg einer derartigen Therapie für den einzelnen Patienten vorhersagen, bislang klinisch nicht etabliert. Als potentielle Prädiktoren beschrieben wurden die Expression von PD‐L1 im Tumorgewebe bzw die Konzentration der LDH im Serum, jedoch vorrangig an selektierten Patientenkohorten aus klinischen Studien.
Das Ziel der aktuellen Subanalyse aus dem prospektiven multizentrischen Register ADOREG/TRIM ist die Korrelation aus Tumorgewebe erhobener molekularer Parameter (Anzahl mutierter Melanom‐assoziierter Gene mittels 30‐Gen Next‐Generation Panel‐Sequenzierung; PD‐L1 Expression mittels Immunhistochemie) mit dem klinischen Verlauf von Patienten mit metastasiertem Melanom aus einem Real‐World‐Setting. Durch den Einsatz eines zentralen Testlabors wurde der Einfluss variabler Testparameter auf die Ergebnisse bestmöglich reduziert.
Zum Zeitpunkt der Auswertung (11/2017) erfüllten 318 Patienten die folgenden Zielkriterien: metastasiertes Stadium, Tumorgewebeprobe geeignet und auswertbar für die molekulare Diagnostik, sowie mindestens eine Verlaufsmeldung vorliegend. Von diesen 318 Patienten hatten 185 eine Behandlung mit anti‐PD1 Antikörpern in Mono‐ oder Kombinationstherapie erhalten (Testkohorte), während 133 Patienten mit anderen oder gar keinen Systemtherapeutika behandelt worden waren (Kontrollkohorte). Es zeigte sich, dass sowohl die Anzahl mutierter Gene aus der Panel‐Sequenzierung (0 vs 1 vs 2 vs ≥3; p = 0.0017), als auch die Expression von PD‐L1 (<5% vs ≥5%; p = 0.012) signifikant mit dem Gesamtüberleben nach anti‐PD1 Therapie assoziiert waren. In der multivariaten Cox‐Hazard‐Regressionsanalyse erwiesen sich beide molekulare Parameter als unabhängige Prädiktoren des Gesamtüberlebens nach anti‐PD1 Therapie. In der Kontrollkohorte ergab sich kein signifikanter Zusammenhang zwischen diesen molekularen Markern und dem Überleben der Patienten (p = 0.71 und p = 0.24).
Unsere Ergebnisse zeigen, dass bei Vorliegen einer Tumorgewebeprobe sowohl die Anzahl mutierter Melanom‐assoziierter Gene als auch die Expression von PD‐L1 unter standardisierten Analysebedingungen als valide Prädiktoren für den Erfolg einer anti‐PD1 Therapie genutzt werden können. Somit wäre eine zukünftige Berücksichtigung dieser molekularen Parameter bei Therapieentscheidungen sinnvoll.
Ugurel S., Horn S., Griewank K. et al. (2018) Die Anzahl mutierter Melanom‐assoziierter Gene und die Expression von PD‐L1 sind unabhängige Prädiktoren der anti‐PD1 Immuntherapie ‐ eine prospektive Registerstudie der ADO (ADOReg/TRIM). Vortrag auf dem 28. Deutschen Hautkrebskongresses (ADO Jahrestagung) 2018
Background:
Clinical cancer registration is increasingly important for healthcare delivery and outcome research in oncology. As compared to clinical trial data, information from clinical routine is often limited regarding the granularity and quality of measures for individual tumor load and distribution.
Methods:
In an effort to implement a robust and useful measure of tumor burden for patients with metastatic melanoma in a German national skin cancer registry (ADOReg) we evaluated the melanoma tumor burden score (MTBS), originally developed for analyzing chemotherapy data in melanoma patients. The MTBS contains a simple categorization of size, number an distribution of metastatic lesions in individual patients. It is aimed at being used on routine radiologic report allowing for a certain level of uncertainty and imprecise quantification of metastatic lesions. Basically, the lesions are categorized per affected organ with respect to number (solitary, few, multiple) and size (≤1cm, >1- 5cm, >5cm). For evaluation of prognostic significance the summary score was calculated and included in univariate and multivariate survival analysis. We performed extensive sensitivity analyses for a variety of different model settings.
Results:
In the primary analysis set we re-evaluated 898 radiologic reports in a total of 235 various chemotherapies in n=128 stage IV melanoma patients. The confirmatory data sets consisted of n=384 stage IV melanoma patients with various treatments including chemotherapy, BRAF inhibitor treatment, and immune checkpoint blockade. MTBS categorization could be applied on routine radiologic reports in the majority of cases (95.7 %). In a multivariate model MTBS remained significantly correlated with outcome when adjusted for age, sex, LDH, and number of metastatic sites. Moreover, change in MTBS correlated to a formal response evaluation according to RECIST.
Conclusions:
The MTBS appears to be a promising tool for meaninful quantification of metastatic tumor load in metastatic melanoma for real life data collection like in clinical cancer registries.
Weichenthal M., Senel G., Both M. et al. (2017) Evaluation of the Melanoma Tumor Burden Score (MTBS) in a real-world setting. Journal of Clinical Oncology. 2017;35(15 suppl):9565. Poster presented at ASCO 2017
https://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.9565
Leiter U., Weichenthal M. (2014) ADOReg - wissenschaftliches Register der Arbeitsgemeinschaft Dermatologische Onkologie. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2014;12:1156-1157. DOI:10.1111/ddg.12556
Study projects of the ADOReg
The following section provides an overview of current study projects.
- Summary of the study objectives: For those patients with advanced (unresectable or metastatic) melanoma the primary objective is to estimate the effectiveness of nivolumab/ipilimumab combination therapy as first line therapy in terms of overall survival (OS) for adult patients with advanced (unresectable or metastatic) melanoma, over a 5-year follow-up period. For those patients receiving adjuvant therapy for resected melanoma the primary objective is to estimate the effectiveness of adjuvant nivolumab therapy, according to the German marketing authorization, in terms of relapse free survival (RFS) in adult patients over a 5-year follow-up period, in real-life conditions in Germany.
- Sponsor: Bristol-Myers Squibb Germany
- Indication(s): Stage III/IV melanoma
- Product: Nivolumab / in combination with Ipilimumab
- Recruitment target: 1050 patients
- Recruitment period: December 2016 to November 2020
- Study period: December 2016 to November 2025
- Summary of the study: In collaboration with ADOReg clinicians, the study aims to describe outcomes of patients treated with pembrolizumab for advanced melanoma in real-world clinical practice. Through leveraging this detailed registry, the study results will advance understanding of pembrolizumab utilization in the real-world and serve as complementary evidence to clinical trials.
- Sponsor: MSD Merck Sharp & Dohme
- Indication(s): Non-resectable, advanced melanoma
- Product: Pembrolizumab
- Recruitment target: 1000 patients
- Recruitment period: August 2015 to December 2019
- Study period: August 2014 to December 2020
- Summary of the study objectives: The objectives of the study are the identification and validation of molecular and clinical novel biomarkers for the outcome of therapy in patients with metastatic melanoma. Clinical and molecular parameters of melanoma patients and their tumors will be recorded in the ADOReg and subsequently correlated with the outcome of subsequent systemic therapies in terms of progression-free survival, overall survival and therapy response. Systemic therapies include all types of currently used therapies (tyrosine kinase inhibitors, immunotherapy and chemotherapy).
- Sponsor: University Hospital Essen
- Indication(s): Metastatic melanoma stage III or IV
- Recruitment target: 1000 patients
- Summary of the study objectives: Primary objectives of the study are to evaluate the duration of therapy response, defined as the time from the first documented response to progression for patients with locally advanced basal cell carcinoma.
Further study objectives are to evaluate the objective response rate, time to treatment response, disease control rate, recurrence rate, time to progression, progression-free survival, overall survival and time to death.
Other pharmacovigilance objectives include describing and reporting adverse events. The incidence, risk factors and the results of serious and non-serious adverse reactions suspected of being associated with vismodegib and those suspected of being associated with other therapies leading to treatment adjustment or discontinuation are described.
- Sponsor: University Hospital Essen
- Indication(s): Local advanced as well as metastatic basal cell carcinoma (laBCC and mBCC)
- Product: Vismodegib
- Recruitment target: 50 patients
- Recruitment period: April 2016 to June 2018
- Study period: April 2016 to December 2021
- Summary of the study objectives: The aim of this case registry study is to collect clinically relevant data on the therapeutic effect of ipilimumab and other therapies after treatment with the anti-PD-1 antibodies nivolumab and pembrolizumab in patients with metastatic melanoma. The primary objective of this study in patients with non-resectable/metastatic melanoma is to describe the objective remission rate and disease control of ipilimumab therapy after failure of PD-1 inhibition. Secondary endpoints of the study are time to next therapy, progression-free survival and overall survival in patients treated with ipilimumab after failure of PD-1 antibody therapy compared to other treatment modalities in Germany. In addition, data on treatment-related adverse events, their effects, safety and treatment pattern after first-line anti-PD-1 therapy are described for BRAF positive and negative patients.
- Sponsor: Elbe Clinics Buxtehude
- Indication(s): Stage III/IV melanoma
- Product: Ipilimumab
- Recruitment target: 200 patients
- Summary of the study objectives: Primary study objectives are the description of relevant patient and tumor characteristics of patients with a MCC diagnosis in Germany and the estimation of prevalence rates and concomitant comorbidities. Furthermore, the MCC-related oncological treatments will be described according to type and therapy line since the initial MCC diagnosis. For patients entering an advanced stage of the disease, the most frequent comorbidities and concomitant medication will also be described, including the pre-medications or medications used to treat undesirable side effects. Especially in the subgroup of immunocompromised patients treated with avelumab, the safety profile, the type of side effects, and the success of the therapy, especially the survival rate overall as well as per therapy line, are investigated. In addition, the safety and efficacy profile of the therapies in immunocompromised patients will be compared to those of immunocompetent patients who were also treated with avelumab. Furthermore, the costs to the health care system are estimated based on the number of MCC-related physician visits, emergency room visits and hospitalizations in patients entering advanced disease stage.
- Sponsor: Merck Healthcare KGaA
- Indication(s): Merkel cell carcinoma
- Product: Avelumab
- Recruitment target: 540
- Recruitment period: April 2019 - Q1 2024
- Study period: Q2 2018-Q4 2024
- EU PAS Registernummer EUPAS25338
Academic projects
Project application to the ADOReg Academic Advisory Board
- Querying the ADOReg database with the aim of processing a specific scientific question (data export in Excel
format) - Application via Word-based application form (link in the download area, see below)
- Assessment and approval by the ADOReg Academic Advisory Board
Timelines
- One application round per year; after the ADO conference
- Deadline for submitting applications at the end of October of the respective year
Eligible applicants
- Scientific staff at the dermatological clinics actively participating in the ADOReg; the application process
should correspond as much as possible to the input effort of the center in the register - The application is limited to one application per project manager and application round; exceptions are possible
after consultation - Multi-center applications, e.g. via the ADO committees are preferred
Criteria for the approval of projects
- Innovative, contemporary and scientifically meaningful questions
- No questions that are the same or similar to those already applied for at ADOReg (see below for an overview of
applied ADOReg projects) - The parameters required for the project should, if possible, already be available in the register; additional
parameters must be requested from the centers retrospectively by the requesting project manager with additional
effort - Similar project applications from different applicants are brought together by the project advisory board and
initiated as cooperation projects - Publications from ADOReg projects must be provided to the Academic Advisory Board for review before submission;
the ADOReg centers involved are to be considered as co-authors, among other things according to the included
number of patients
Tumour entity: Malignant Melanoma
Main applicant/contact (further applicants): Zimmer, Lisa (Zaremba, Anne)
Tumour entity: Squamous Cell Carcinoma
Main applicant/contact (further applicants): Tietze, Julia (Weichenthal, Michael)
Tumour entity: Malignant Melanoma
Main applicant/contact (further applicants): Tietze, Julia (Weichenthal, Michael)
Tumour entity: Malignant Melanoma
Main applicant/contact (further applicants): Tietze, Julia (Weichenthal, Michael)
Tumour entity: Malignant Melanoma
Main applicant/contact (further applicants): Schilling, Bastian (Kreft, Sophia)
Tumour entity: Malignant Melanoma
Main applicant/contact (further applicants): Rösch, Alexander (Hinney, Anke; Ugurel, Selma)
Tumour entity: Malignant Melanoma
Main applicant/contact (further applicants): Pföhler, Claudia (Ugurel, Selma)
Tumour entity: Malignant Melanoma
Main applicant/contact (further applicants): Mohr, Peter (Weichenthal, Michael)
Tumour entity: Basal Cell Carcinoma
Main applicant/contact (further applicants): Leiter, Ulrike
Tumour entity: Malignant Melanoma
Main applicant/contact (further applicants): Gutzmer, Ralf
Tumour entity: Malignant Melanoma
Main applicant/contact (further applicants): Griewank, Klaus (Livingstone, Elisabeth; Lodde, Georg)
Tumour entity: Malignant Melanoma
Main applicant/contact (further applicants): Franklin, Cindy (Ugurel, Selma)
Tumour entity: Merkel Cell Carcinoma
Main applicant/contact (further applicants): Becker, Jürgen (Ugurel, Selma)
Tumour entity: Malignant Melanoma
Main applicant/contact (further applicants): Gutzmer, Ralf
Tumour entity: Malignant Melanoma
Main applicant/contact (further applicants): Heppt, Markus; Schlaak, Max
Tumour entity: Malignant Melanoma
Main applicant/contact (further applicants): Loquai, Carmen (Stege, Henner)
Media library/downloads
ADOReg brochure
ADOReg_brochure_June2019.pdf
ADOReg committee structure
ADOReg_committee_structure_May2020.pdf
Application form for conducting an academic project
Application-form-for-conducting-an-academic-project-EN.docx
Process and contact for academic projects
Process_and_contact_for_academic_projects_June2020.pdf
Contact persons
The ADOReg Steering Group makes decisions on all relevant issues concerning the operation and further development of the Registry, including decisions on the implementation of contract research and academic research.

Prof. Dr. med. Dirk Schadendorf
Universitätsklinikum Essen, Director of the Department of Dermatology, Venerology and Allergology
dirk.schadendorf@uk-essen.de
+49 (0) 201 723 4342

Prof. Dr. med. Michael Weichenthal
Universitätsklinikum Schleswig-Holstein, Campus Kiel, Senior Physician & Head of the Epidemiology and Statistics Group at the Department of Dermatology, Venerology and Allergology
mweichenthal@dermatology.uni-kiel.de
+49 (0) 431 5971537

Prof. Dr. med. Ralf Gutzmer
Medizinische Hochschule Hannover (MHH), Head of the Skin Tumour Centre Hannover (HTZH)
Gutzmer.Ralf@mh-hannover.de
+49 (0) 511 5320

Dr. Gabriele Haas
IQVIA, Senior Principal, Real World Solutions
gabriele.haas@iqvia.com
+49 (0) 69 6604 4883
The registry operations and data collection software are managed by a representative of the ADO and the responsible IQVIA employee. The aim and purpose of this group is to maintain and optimise the basic register.
The members make an important contribution to register coordination and further development. Their work has made the establishment of the register possible and ensured the scientific and high quality of the register. If you are interested in an academic cooperation with ADOReg, please contact the persons listed below.

Prof. Dr. med. Ulrike Leiter-Stöppke
Management of the outpatient clinic for epithelial and rare skin tumours
Universitäts-Hautklinik Tübingen
ulrike.leiter@med.uni-tuebingen.de
Tel. +49 (0) 7071 298 4599
If you have any questions, please contact our support team on +49 (0) 3342 42689-22 or send an e-mail to support@adoreg.de.
The Academic Advisory Board is appointed by the ADOReg steering group in agreement with the Scientific Advisory Board. The Academic Advisory Board examines and coordinates applications for academic projects. Please also refer to the downloads section for the ADOReg’s academic project process and request form.
Primary contact:

Prof. Dr. med. Selma Ugurel
Senior physician at the clinic for dermatology, venerology and allergology
selma.ugurel@uk-essen.de
+49 (0) 201 723 4714

Prof. Dr. med. Ulrike Leiter-Stöppke
Management of the outpatient clinic for epithelial and rare skin tumours, Universitäts-Hautklinik Tübingen
ulrike.leiter@med.uni-tuebingen.de
Tel. +49 (0) 7071 298 4599
Contact
Do you have a general request or a specific inquiry? Please feel free to contact us and send us an e-mail to support@adoreg.de.
Support: +49 30 800 930 8-50
Office: +49 30 800 930 8-30